Corrective and Preventative Actions Basics
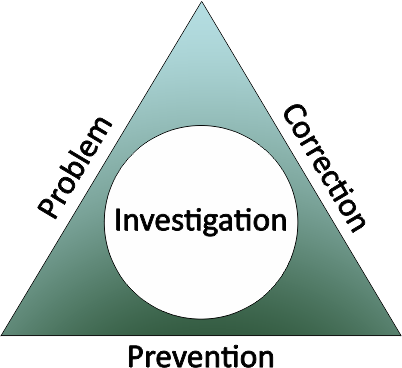
Corrective and Preventive Action is the methodology of tackling non-conforming events, materials or processes by tracking, investigating, correcting and then acting to prevent future problems, as part of your Quality Management System. The goal is to develop and support a culture of continuous improvement as an element of management commitment to stakeholders.
The most successful CAPA (corrective and preventative action) systems are those that have a ton of staff engagement because they are easy to use, and stakeholders really understand and have experienced the benefits achieved from dedication to continuous improvement.
Terms and Definitions to Describe CAPA Processes
These Corrective and Preventive Basics terms and definitions are frequently used in describing and operating the CAPA process. Explanations of the goals and actions of the CAPA system helps staff better understand how to comply with requirements, meet operational goals, and improve employee satisfaction.
The Role of CAPA in Quality Management
What does the corrective and preventative action (CAPA) system do?
The corrective and preventative action system tracks and manages the tasks, staff and resources engaged in the effort to achieve continuous improvement, whether that is because of compliance needs or management policy.
What information is maintained in the CAPA System?
A CAPA system contains information on all discrepancies encountered and efforts made to correct, investigate and verify the specified preventative actions. A critical element of a successful system is the ability to monitor all data and easily provide management reporting.
What is a CAPA Plan?
A CAPA plan is a corrective and preventive action plan, designed to identify and rectify specific issues and ensure they aren’t repeated.
What is CAPA in Quality Management?
CAPA (Corrective and Preventative Action) is the part of your Quality Management System that is focused on achieving continuous improvement. It is based on recognizing that the best way to prevent future problems or discrepancies is to dedicate resources to identifying, researching and solving current issues.
What is CAPA management?
It is the administrative practice of running your Corrective and Preventative Action system to ensure you are monitoring and verifying completion of the tasks of capturing discrepancies, taking appropriate corrective steps to satisfy risk and customer needs, then applying formal methods to the effort of investigating root causes of these failure so that problems are solved or eliminated through the application of preventative actions.
What are the risks of poor CAPA Management?
Poor performance of your CAPA system often results in failure to meet ISO standards and to pass audits or obtain other certifications. The risk from poor management of a CAPA system arises if the necessary closed loop processes do not occur in a timely manner or are missed completely. This means that adequate corrective or preventative steps are not occurring, and that issues and related costs continue to repeat and increase over time.
Managing Improvement Continuously
What is Improvement Management?
Improvement management is a strategy for achieving better outcomes for stakeholders through a formal process of investigation of problems or issues that have been classified as impediments to success and implementing changes designed to avoid or eliminate the problems. These efforts are often documented and tracked using a CAPA system.
What is Continuous Improvement?
Continuous Improvement is an operational strategy that constantly seeks and then executes changes designed to achieve better outcomes. It is based on the belief few processes or products are optimized, therefore there is always opportunity to achieve better outcomes by identifying and executing useful changes.
What are the benefits of Continuous Improvement?
The benefits of continuous improvement include increased profitability, higher levels of customer satisfaction and greater employee loyalty. The proactive addressing of potential causes of harm helps organizations avoid costs to fix errors and reduces stress on staff and customers.
Understanding Non-Conformances
What is a Non-Conformance report?
A non-conformance report is a document that records information about an event, material or process that has not met the stated standards or requirements. It is typically the first formal step in a CAPA (corrective or preventative action) system. The information needed for a complete report will vary by industry or application.
What should a non-conformance report contain?
The most important information is a clear statement of how the non-conformance is manifested which includes specifics about the standard or requirement that is not being met. Identification of the urgency of a need to respond in a corrective manner is an important guide for establishing priority of follow up activities, this can include dates for completion, risk evaluations or similar categorizations and estimate of financial impact. Establishing an effective tracking mechanism requires clear assignment of responsibility for further action. Evidence data such as locations, reporting personnel, part numbers, vendor id, customer id, lot and/or serial numbers are critical for complete analysis and to support additional investigation. It is important to note that many non-conformances do not generate simple one to one actions and outcomes. Frequently an appropriate level of response to a non-conformance may require multiple actions or investigations be launched.
How to Identify the Source of Problems
What is a root cause analysis?
A root cause analysis is an effort to diagnose and identify the source(s) of a problem to a degree of confidence that the cause is correctly identified. Often when a root cause is defined the next step is to determine what changes need to occur to eliminate the cause as a potential source of future problems. These elimination steps area generally managed through preventive action documentation.
What is "5 Whys"?
This is an iterative method of formally seeking the source of a problem by inquiring “why” an outcome occurred and then repeating that investigation, or “why”, for each supplied reason until a final source cause has been located. The goal is to encourage persistence of inquiry and achieve confidence in the resulting analysis of cause.
Corrective and Preventive Action Documentation Explained
What is preventative action in continuous improvement?
Preventative action in continuous improvement is the direction, upon completion of diagnosis or investigation, to execute changes or adaptions to processes or performance in order to eliminate cause(s) that have the potential to create non-compliant outcomes.
What is corrective action in continuous improvement?
Corrective actions in continuous improvement are directions to fix or adjust performance in order to obtain compliant results in circumstances where that compliance was not achieved as expected. Generally, they are immediate or short-term fixes with a view to mitigating impacts of failures. They will later become part of a broader preventative action initiative to ensure failure won’t be repeated.
What is the difference between corrective and preventative actions?
The primary difference is that corrective actions are solely issued in response to current non-compliances, while preventative actions are directions to make changes that remove the potential of future errors. Corrective actions are current directions for existing non-compliances and preventative actions focus on changes that affect future outcomes.
Supplier and Customer Requirements
What is Supplier Qualification?
Supplier or vendor qualification is a process of examining products and processes of suppliers to be sure that they conform to the requirements defined for materials obtained. Conformance to FDA or other regulatory standards, such as ISO, requires defined processes exist for timely evaluation and monitoring of supplier performance.
What is a Deviation Request?
In quality systems, when outcomes do not meet requirements in a fashion that is consider no or low risk, requests can be made to accept a deviation from requirements as an adaptation to fulfilling those requirements. Frequently, such requests will be from vendors to customers.
What is a Customer Complaint?
A customer complaint occurs when customers are not satisfied with products or services and they identify that dissatisfaction directly, with or without an accompanying request, to solve for that dissatisfaction.
What is change control?
Change control is a method of managing modifications to the tools, such as the documents, data or processes that direct actions or information in an organization, so that a known approved state of each tool is maintained even when the tool is undergoing revision or change.
Get Started Now
Contact Information
SALES
sales@qualityessentialssuite.com
+1 (866) 949-9504, ext 811
SUPPORT
support@qualityessentialssuite.com
+1 (866) 949-9504, ext 2
